Laboratory work
Most of our analytical work is carried out in the labs of the Bristol Isotope Group.
Coral and Deck Lab:
Collection and processing of deep-sea corals
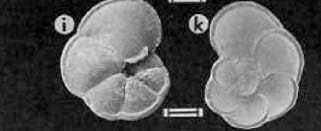
A major goal of this expedition is to learn about and from deep-sea corals. In order to learn about deep-sea corals, it is crucial to identify modern deep-sea coral habitats and environmental factors controlling such habitats. In addition, we also dare to learn from deep-sea corals since they can tell us a lot about past ocean conditions. Therefore, it is essential to collect specimen from the seafloor at first! In case of deep-sea corals “seafloor” usually means underwater mountains, so-called “seamounts” being up to several kilometers high. During this expedition we use the Remotely Operated Vehicle (ROV) ISIS giving us the opportunity of being so that we can collect the most appealing samples from the deep sea.
After the ROV dive, the recovered samples (i.e., besides corals also sponges, worms and much more) are transferred into the constant temperature lab (cold!) where live and fossil (i.e., dead and old) are split. The fossil corals are moved to the deck lab where they are placed in trays for drying – accompanied by some spongy deep-sea friends we get a subtle smell of the deep sea in our lab. Once dried, the fossil corals are identified so that they can get a dedicated unique “fossil-ID”. Followed by a photo shooting, the coral samples are eventually bagged.

For some corals it follows the preparation for further analyses. More precisely, we cut the poor things in pieces in order to analyze the chemistry of their skeletons. Coral skeletons are precipitated from the seawater they grew in, and hence incorporate past ocean chemistry. However, it is crucial to determine the age of a fossil coral at first in order to do targeted further chemical analyses for a particular period of time. In this case, our “multi-proxy” approach involves different elemental and isotopical analyses in order to reconstruct potential past (glacial-interglacial) ocean variability of temperature, marine carbon cycling and ocean circulation.
Age determination and further chemical analyses require (clean) lab facilities which cannot be provided on the ship. The chemical analyses usually include coral digestion in acid for chemical separation of different elements in clean lab facilities onshore in order to measure small variations in coral elemental and isotopical composition (i.e., their chemistry) on high-precision mass spectrometers.
Water Sampling Lab:
This laboratory opens straight onto the sea! The CTD lives in here so that it can be lowered directly out into the water. The CTD measures conductivity, temperature and depth in the seawater. CTDs can also measure other properties such as dissolved oxygen and chlorophyll fluorescence (which shows how many phytoplankton are in the water). The CTD sits in a framework beneath a ring of large bottles known as a rosette or carousel. The bottles are called Niskin bottles, and have an opening at the top and bottom which an be closed simultaneously to trap seawater inside. Each bottle can be closed at a specified depth, providing scientists with water samples from a range of depths under the sea.

Once the apparatus is brought back up onto the ship, scientists can take seawater from the bottles in the rosette and measure many different properties of the seawater. Some measurements have to be done as quickly as possible, or else the seawater will be affected by the atmosphere and will no longer provide good results. This means that there is a certain order in which samples are collected from the bottles for analysis:
- Dissolved oxygen
- Carbonate chemistry
- Radiocarbon
- Nutrients
- Oxygen isotopes
- Silicon isotopes
- Nitrogen isotopes
- Radiogenic isotopes
- Trace metals
- Salinity
- Particulate organic carbon
- Particulate organic nitrogen
Each seawater sample is treated differently to measure the different properties listed above. For example, the seawater measured for dissolved oxygen has reagents added to it which turns the sample orange. The chemicals bind to the oxygen in the water creating this orange colour. Later, the samples can be titrated to find out how much dissolved oxygen they contained.

Foraminifera Biostratigraphy:
Planktonic foraminifera are unicellular zooplankton which live free floating in the open ocean. They make their skeletons (tests) out of calcium carbonate (CaCO3) and when they die, they sink to the sea floor and their tests become preserved in deep-sea sediments. These preserved microfossils are widely used as a tool to determine the relative dates of marine pelagic sediment cores, a practice known as biostratigraphy. There are many reasons why planktic forams are ideal for biostratigraphy: they are morphologically distinct and diverse, they are highly abundant, globally distributed and they evolve rapidly. The spatial variability of foraminifera in the oceans is controlled by nutrient availability, sea surface temperature, salinity and turbidity and each species is adapted to inhabit a particular combination of these factors. As a consequence, past shifts in climate can be correlated with successional changes in species abundance.
The species of foraminifera that we are most interested in on the TROPICS cruise is Globorotalia menardii. Menardii is interesting because its abundance has been highly responsive to changes in climate during the Pleistocene. The presence or absence of this species characterizes well-defined climate zones in the Pleistocene which are widespread in the equatorial Atlantic and Caribbean. Generally, this tropical species appears more abundant in warm interglacial periods, and is rare or completely absent during cold, glacial periiods. Thus, analysing the abundance of G. menardii relative to all other species in a sediment core can provide a ‘quick and dirty’ tool to estimate sediment ages.